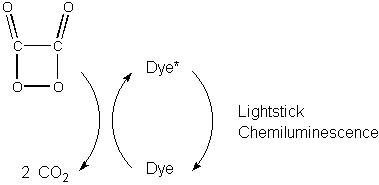

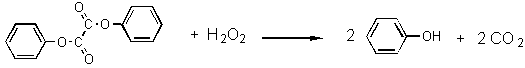
An intermediate is formed during the oxidation that has a high
energy
four membered ring. The breakdown of this intermediate passes energy to
a dye molecule, placing the dye molecule in an excited electronic
state.
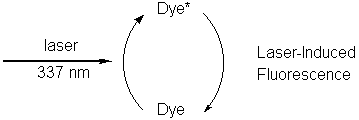
The dye molecule fluoresces, returning to its ground state, thus
producing
the light from the lightstick.

If the dye molecule can be placed in its excited state through some
other means, such as laser excitation the lightstick will produce the
same
light.